Sustainability 101: Climate Change (Part 1: Ice Cores)
Don't try reading this line: “A Computational Determination of the Lowest Energy Electronic and Geometric States of First Row Transition Metal Dioxygen Dications”*
That’s the title of the single peer-reviewed publication I have to my name. Even the title likely means next to nothing unless you’re a chemist. This is a great example of how academics are notorious for creating language that is exclusive. As a student and researcher I liked to go up to presenters at research conferences, read their title aloud, then kindly ask them to tell me what it meant in plain English.
Yes, I have always been a little bit cheeky. Although this is a great example of how a key skill for anyone is taking the most complicated information and making it palatable and digestible to somebody else.
Climate Change Misconceptions
Of course, one of the first places I go after saying that I would make things easy to understand is one of the most complex concepts. What can I say? I love a challenge.
When I say that I am a former climate change scientist, I get a broad range of reactions – especially in my home country of the United States. I have had a gentleman from a half-generation above me challenge me on the entire concept of “global warming” because when he was learning about it, it was the scary specter of “global cooling” – therefore scientists clearly had no grasp on what reality was if we have swung so far so quickly. A retired CEO asked me if I thought that the concept of climate change was overblown, or even made up, to provide a platform to undermine the free market for political reasons. I’ve had a grade school teacher in New York applaud my profession, and express her hope that I can influence a quick and complete phase-out of all fossil fuels.
Clearly there are some misconceptions out there about what climate change is, what the causes are, what dangers it poses, and what opportunities we have to change its path forward.
Climate Time Capsules - How do we know ancient climate?
I was lucky enough in graduate school to be part of a program where we brought university Environmental Science resources to K-12 classrooms. One of the items that we could bring with us was a real piece of an ice core. This is one of the key sources of information that climate scientists have to show the past 800,000 years of climate trends. For reference, modern humans have existed as a species for about 50,000 years.
If you live in northern climates you’re familiar with snow. As snow falls over the course of the winter season, softer fluffy snow accumulates, and the older snow underneath compresses and gets crusty. I grew up in New England, so I never saw more than maybe 5 or 6 feet (1.5 to 1.8 meters) on the ground at once. However, if you get a significant amount of accumulation the weight of the newer snow actually compresses the older snow into ice. All of the air pockets between the former snowflakes are then trapped as tiny time capsules. The above drawing illustrates this process.
Ice cores have annual rings in them, very similar to tree rings. The ice core that I used to bring into classrooms was kept in a deep-freeze cooler and had a light fitted behind the core so that students could easily see the rings. These relate to rapid annual winter accumulation and summertime partial-melt. The arrows in the below picture show the lighter summertime layers.
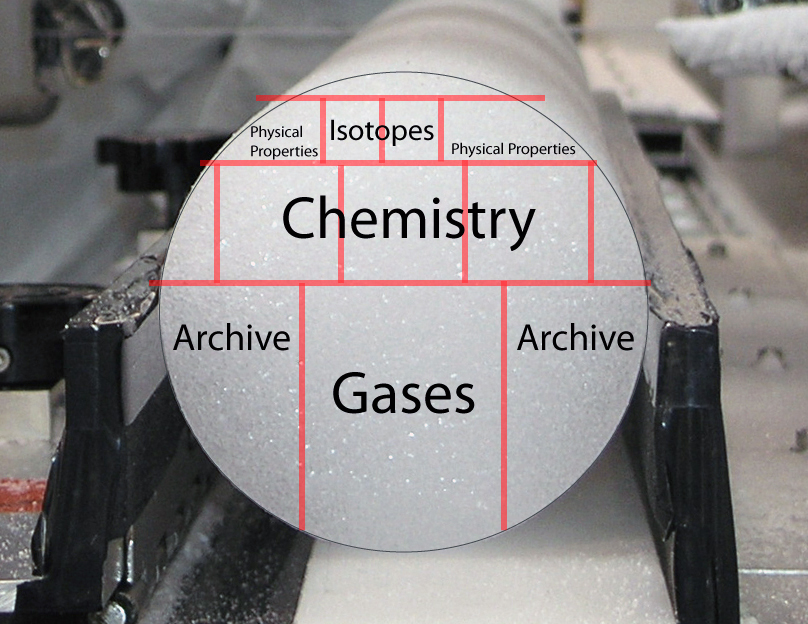
Scientists cut slices of the cores and analyze every aspect of them. I won’t get into the weeds on the various aspects, but will give a brief rundown of the two big ones.
Gasses: Climate scientists place a section of the core in a vacuum and then crush, melt, or grate the core sample. As I wrote above, there are many tiny bubbles of ancient air in these cores. As this air is released, it is analyzed. From this we know what the atmospheric composition was like years before now.
Isotopes:
This can be used for time dating (think carbon or uranium dating) from dust trapped in the cores.
Historic temperature can be calculated with chemistry. Think back to general chemistry class and the periodic table. Oxygen usually exists as 16O, having 8 neutrons and 8 protons. This is the state of oxygen 99.76% of the time. However, there are two other stable (non-radioactive) oxygen isotopes. The most abundant of these (0.20%) is 18O, with 8 protons and 10 neutrons. Water (H2O) can use any oxygen isotope. It takes more energy to evaporate water with the heavier O, and this heavier 18O water is also rained out of the atmosphere more quickly. Scientists have observed that in warmer temperatures, more heavy water reaches the arctic – with a measurable ratio of 16O to 18O that changes with temperature. This makes sense, because higher temperatures mean more heat energy, and more energy to lift the heavy water. Using this information and global standards scientists have an “isotope thermometer” using 16O:18O in the ice cores to say what the temperature was when the ice was deposited as snow.
This now gives us a basic understanding of the information that ice cores can provide. With this knowledge, we can look at an ice core record of the past. The below graph is a depiction of the temperature difference from present day and CO2 concentration over time.
Notice the “saw tooth” pattern. This is a standard cyclical pattern of heating and cooling over timespans that go way beyond even the existence of humans. These patterns are attributed to natural cycles of the movement of Earth. These are called the Milankovitch cycles, and are related to the direction and tilt of the Earth’s axis (like a top or gyroscope that wobbles a bit) and the change in shape of the Earth’s orbit around the sun. Explaining astrophysics in detail is a little beyond the scope of this series, so if you are interested in learning more please do.
Due to the historic climate change data, there was some panic in the late 20th century that we were approaching a period of rapid global cooling. After all, the record said that this was imminent if we followed the historic trend. From the above chart, you can see that if history repeats itself we are due for a crash into an ice age. However, that natural cooling never happened. Just the opposite, actually.
To be Continued...
*PS: In case you were wondering, the plain-English explanation of my publication is: I used a computer program to look at how Oxygen (O2) best combines with metals from the first row of the flat part of the periodic table (Scandium to Zinc). Think of it like a computer model of a Tinker Toy – finding the best way O2 and the metal fit together without being unstable.
Thank you for joining me for this Sustainability 101 explanation. There is a lot of ground to cover in this series, so please join me for upcoming articles on subjects like climate change and science-based targets, the UN’s Sustainable Development Goals, and solutions like RECs, carbon offsets, and carbon taxes.